By Andreas Bock Michelsen
This is the first part in a series on the fundamental physical concepts involved in talking about energy.
The anticipatory click of a seat belt, the now very redundant “no smoking” light, ritualistic security announcements from the crew, and finally the sucking feeling in your stomach as the flight takes off, carrying you to faraway lands. Getting on a flight might seem a distant memory to most these days, but when it comes to price and speed, flying is the far superior mode of transportation at most medium and long ranges. This superiority comes at a steep environmental cost, though. Aviation accounts for as much as 2% of global carbon dioxide emissions, and 12 % of the personal yearly carbon footprint of an average UK citizen, or half the total emissions from all electricity use.
While investments are being made in Europe to provide medium range alternatives in the form of highly fuel-efficient night trains, there is no beating aviation at long distance. The question is then: can airplane emissions be reduced enough for flights to survive in a net zero-emission world?
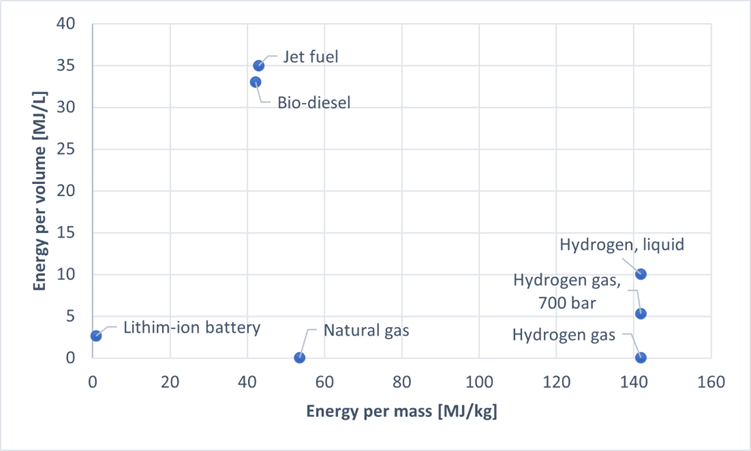
In addressing this question, for simplicity we will only consider the emissions from actual air travel, not from the production of planes. To understand the challenges in achieving emission-free flight, we need to understand what constitutes a good energy source in the specific context of transport. An airplane cannot be connected to cables during its flight, so it needs to bring some sort of fuel. The heavier the airplane is, the more power is needed to get it going, so ideally the fuel should be as light as possible. The key word then is specific energy, also known as energy density. A fuel source with high specific energy can provide a lot of energy for each kilogram of fuel you bring, so that is what we want for our airplanes. The big issue, then, is that carbon-based fuel is both relatively cheap and has a comparatively high specific energy, making it the ideal aviation fuel. If we ignore environmental impact, that is.
An immediate alternate candidate presents itself when we consider the similar issue of car travel. Great strides are being made in electrifying cars, so how about doing the same in planes? If we can install a big battery in the airplane, we can charge it from renewable sources and get rid of carbon emissions from the flight itself. The problem, as you may have already guessed, is the specific energy. A lithium-ion battery has a specific energy that is orders of magnitude less than that of jet fuel, an insurmountable gap which will not be closed by any currently viable technological advances. That is not to say that battery powered flights have no role in the future – there are currently proposals for hybrid airplanes, where take-off and landing are done with regular fuel, and the much less power-intensive mid-air cruise in between is handled by the battery. After all, while cruising, the airplane mostly needs to defeat the friction from the air, with the heavy lifting already taken care of.
The battery issue reveals another important detail for choosing aviation fuel. To release the energy stored in fuel or a battery, we use the chemical process of oxidization. For the battery, this process is a completely internal process of moving electrons around the fluid contained within. For jet fuel, however, oxidization happens by mixing the fuel with air and letting it combust. We say that the battery has to carry its own oxidizer, while regular jet fuel can use the surrounding air for energy release. This makes batteries a relatively heavy source of energy, but here we also arrive at the essence of our problem: since jet fuel is based on carbon, C, and is oxidized with oxygen, O, from the air, the result of the process is carbon dioxide, CO2, the primary greenhouse gas.
We are looking for a clean but combustible fuel, then. Several candidates exist, including natural gas and biofuel. But one candidate stands out with more than three times the specific energy of jet fuel and which can be oxidized in open air without pollution: hydrogen. Hydrogen can be cleanly produced from water, H2O, through electrolysis, where electricity (ideally from a renewable source) is used to separate the hydrogen and oxygen again. Just like jet fuel, the hydrogen can be mixed with air and combusted, but this time the only result is water vapor. Hydrogen is no magical solution, however, and it has a number of important problems which need to be resolved before becoming a viable and economical fuel choice. A major problem of using hydrogen as fuel is storage – it takes up a lot of space, even when heavily compressed, see Fig. 1. Solving this problem is far from impossible. But while the cost of jet fuel is rising due to dwindling supply, it might not be until the 2030’s before hydrogen is a commercially viable alternative.
The key to sustainable aviation is the development of a renewable fuel, preferably combustible, with a high specific energy. Several proposals exist which bring this within technological reach, but policy and the markets need to give the technology a hand if we are to get there in time.
Featured Image: “British Airways Boeing 747 contrail” by revedavion.com is licensed under CC BY-SA 2.0